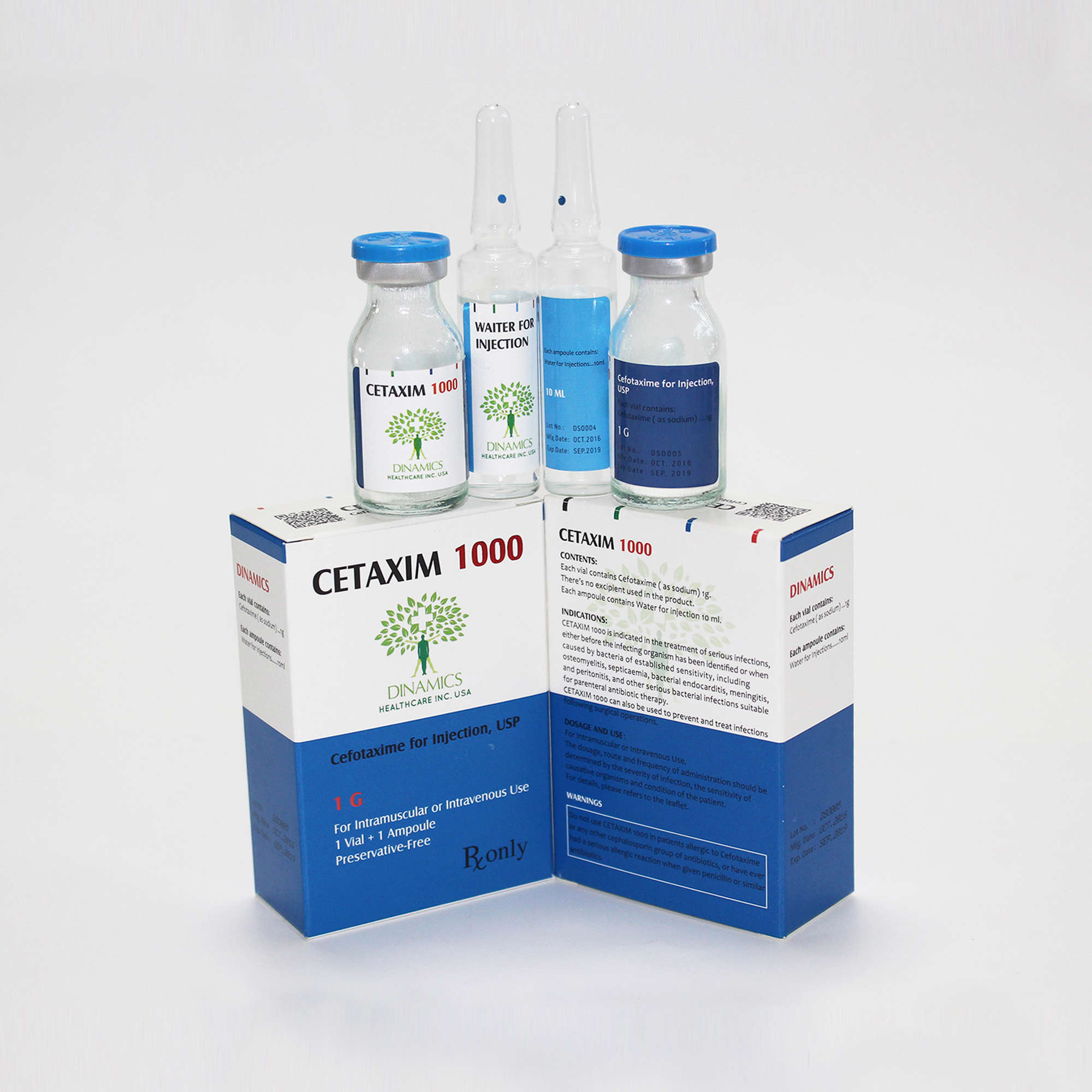
Cefotaxime for Injection, USP
QUICK DETAILS:
CETAXIM 1000 Contents:
• Each vial contains Cefotaxime ( as sodium) 1g as the active ingredient.
• There’s no excipient used in the product.
• Each ampoule contains 10ml of water for injection as the solvent.
CETAXIM 1000 Character:
CETAXIM 1000 is a white to pale yellow crystalline powder.
CETAXIM 1000 Pack:
1 vial and 1 ampoule per box.
DESCRIPTION
1. What CETAXIM 1000 is and what it is used for
Cefotaxime, the active ingredient of CETAXIM 1000, is an antibiotic. It belongs to a group of antibiotics that are called cephalosporins. These types of antibiotic are similar to penicillin.
CETAXIM 1000 is indicated in the treatment of serious infections, either before the infecting organism has been identified or when caused by bacteria of established sensitivity, including osteomyelitis, septicaemia, bacterial endocarditis, meningitis, and peritonitis, and other serious bacterial infections suitable for parenteral antibiotic therapy.
CETAXIM 1000 can also be used to prevent and treat infections following surgical operations.
2. What you need to know before you take CETAXIM 1000
CETAXIM 1000 should not be given if:
• you are allergic (hypersensitive) to cefotaxime
• you are allergic to cephalosporin group of antibiotics
• you have ever had a serious allergic reaction when given penicillin or similar antibiotics
Before you are given Cefotaxime injection
You must tell the doctor or nurse if any of the following apply to you:
• you have a history of allergies or asthma
• you are on a low sodium diet
• you have a heart or kidney disorder
• you are having any blood or urine tests
• you have a history of gastro-intestinal problems e.g. colitis, which causes diarrhoea containing blood.
Taking other medicines
Tell the doctor or nurse if you are taking any of the following medicines:
• the contraceptive pill (in which case you will need to take extra contraceptive precautions such as using a condom)
• any diuretic medicine (“water tablets”) e.g. furosemide
• another antibiotic e.g. chloramphenicol or aminoglycoside antibiotics
• probenecid (for gout)
Please tell your doctor if you are taking, or have recently taken, any other medicines including any that you may have bought without a prescription.
Pregnancy and lactation
Pregnancy: The safety of cefotaxime has not been established in human pregnancy. Cefotaxime crosses the placental barrier. Therefore, cefotaxime should not be used during pregnancy unless the anticipated benefit outweighs any potential risks.
Lactation: Cefotaxime passes into human breast milk in small amounts and is usually compatible with breast feeding, but careful monitoring of the infant is recommended.
Effects on the physiological intestinal flora of the breast-fed infant leading to diarrhoea, colonisation by yeast-like fungi, and sensitisation of the infant cannot be excluded.
Therefore, a decision must be made whether to discontinue breast-feeding or to discontinue therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman. For the sake of safety, this medicine should not be used in pregnancy and breast-feeding women unless absolutely indicated.
You should let your doctor or nurse know if you are pregnant or wish to become pregnant or are breast-feeding before this medicine is administered.
Driving and using machines
If you are given high doses of cefotaxime, you may feel dizzy/drowsy or fall asleep or experience convulsions (fits) or unusual body movements. If this happens, you should not drive or operate machinery.
Important information about some of the ingredients of Cefotaxime injection
Cefotaxime Injection contains approximately 50mg (2.2 mmol) of sodium per 1g dose. This should be taken into consideration by patients on a controlled sodium diet. Tell your doctor or nurse if you are on a low sodium diet.
DIRECTION
1.How to take CETAXIM 1000
CETAXIM 1000 may be administered intravenously by bolus injection or by infusion, or by intramuscular injection. The dosage, route and frequency of administration should be determined by the severity of infection, the sensitivity of causative organisms and condition of the patient. Therapy may be initiated before the results of sensitivity tests are known.
Adults:
The recommended dosage for mild to moderate infections is 1g 12 hourly. However, dosage may be varied according to the severity of the infection, sensitivity of causative organisms and condition of the patient. Therapy may be initiated before the results of sensitivity tests are known. In severe infections dosage may be increased up to 12g daily given in three or four divided doses. For infections caused by sensitive Pseudomonas species daily doses of greater than 6g will usually be required.
Children:
The usual dosage range is 100-150mg/kg/day in two to four divided doses. However, in very severe infection doses of up to 200mg/kg/day may be required.
Neonates:
The recommended dosage is 50mg/kg/day in two to four divided doses. In severe infections 150-200mg/kg/day, in divided doses, have been given.
Dosage in renal impairment:
Because of extra-renal elimination, it is only necessary to reduce the dosage of cefotaxime in severe renal failure (GFR <5ml/min = serum creatinine approximately 751 micromol/litre). After an initial loading dose of 1g, daily dose should be halved without change in the frequency of dosing, i.e. 1g twelve hourly becomes 0.5g twelve hourly, 1g eight hourly becomes 0.5g eight hourly, 2g eight hourly becomes 1g eight hourly etc. As in all other patients, dosage may require further adjustment according to the course of the infection and the general condition of the patient.
Dosage in hepatic impairment:
No dosage adjustment is required.
Intramuscular injection: 1g Cefotaxime should be dissolved in 3ml of Water for Injection. Afterwards the injection should take place deep into the gluteal muscle.
Intravenous injection: 1g Cefotaxime should be dissolved in 10ml of Water for Injection. The injection should be administered over 3 - 5 minutes, directly into the vein.
Intravenous infusion: 1g of Cefotaxime should be reconstituted with 50 or 100ml of 0.9% Sodium Chloride Injection or 5% Dextrose Injection. The prepared infusion may be administered over 20-60 minutes.
2. Possible side effects
Like all medicines, CETAXIM 1000 can cause side effects, although not everybody gets them.
As with other antibiotics, some people find they have an allergy to it.
Tell your doctor immediately if any of the following rare symptoms occur:
•Sudden wheeziness and tightness of chest
•Swelling of eyelids, face, lips or throat
•Skin lumps or “hives” (nettle rash)
•Severe skin rashes with itching
•Serious illness with blistering of the skin, mouth, eyes and genitals
•Loss of consciousness, abnormal movements or convulsions (fits) Antibiotic treatment can affect the normal bacteria in the gut, causing new infection (colitis). You should tell your doctor immediately if you develop diarrhoea.
The following side effects may occur in some patients treated with Cefotaxime injection. Tell your doctor if any become troublesome:
Very common side effects (probably affecting more than 1 in 10 people)
• pain at the injection site
Uncommon side effects (probably affecting less than 1 in 100 patients)
•reduction in blood platelets which increases risk of bruising or bleeding
•reduction in number of white blood cells which makes infections more likely
•increase in number of white blood cells
•fever
•increase in liver enzymes and/or bilirubin
•kidney problems
•skin rash, itching, ‘hives’ (nettle rash)
•difficulty breathing
•convulsions (fits)
•diarrhoea
•redness and swelling at injection site
Side effects occurring with unknown frequency
•secondary infections
•serious allergic reactions which may cause difficulty in breathing or dizziness
•serious allergic reaction which causes swelling of the face or throat
•difficulty in breathing or wheezing
•headache
•dizziness
•loss of consciousness, abnormal movements
•feeling sick (nausea)
•being sick (vomiting)
•stomach pains
•diarrhoea containing blood
•inflammation of the liver (hepatitis)
•skin and whites of the eyes turn yellow (jaundice)
•painful joints
•irregular heart rhythm
•inflammation of the kidneys which may cause dark discolouration of urine, cloudy/bloody urine, or any change in your urine output.
Treatment with high doses of Cefotaxime, particularly in patients with kidney problems, has been known to cause loss of consciousness, abnormal movements and convulsions (“fits”).
INGREDIENTS
Contents of the pack and other information
What CETAXIM 1000 contains
•Each vial contains Cefotaxime ( as sodium) 1g as the active ingredient.
•There’s no excipient used in the product.
•Each ampoule contains 10ml of water for injection as the solvent.
What CETAXIM 1000 looks like and contents of the pack
CETAXIM 1000 is a white to pale yellow crystalline powder. 1 vial and 1 ampoule per box.
STORE
How to store CETAXIM 1000
CETAXIM 1000 must not be used after the expiry date ‘EXP’ shown on the container.
Keep out of the reach and sight of children.
Do not store above 25°C. Keep the vial in the outer carton. After reconstitution: Store at 2-8°C. Do not freeze.