Ketorolac Tromethamine Injection, USP
QUICK DETAILS:
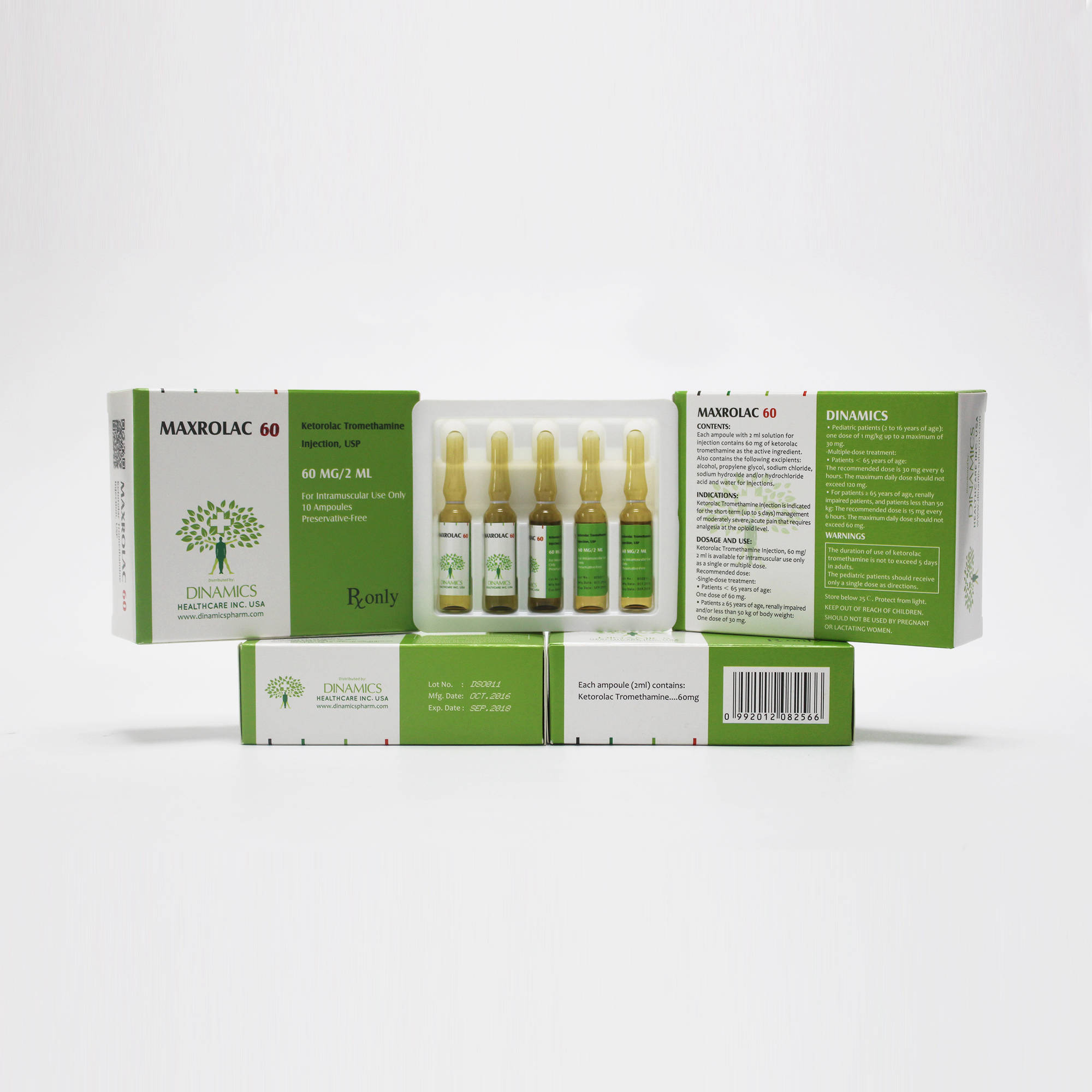
MAXROLAC 60 Contents:
•Each ampoule of the active substance is Ketorolac Tromethamine.
•The other ingredients are: alcohol, propylene glycol, sodium chloride, sodium hydroxide and/or hydrochloride acid and water for injections.
MAXROLAC 60 Character:
•MAXROLAC 60 is a clear and colorless or slightly yellow solution in color, free from visible particles.
MAXROLAC 60 Pack:
Available in 1 mL ampoules, 5 ampoules per tray, 2 trays per carton.
DESCRIPTION
1. What MAXROLAC 60 is and what it is used for
MAXROLAC 60 contains the active ingredient of ketorolac tromethamine, which is a member of the pyrrolo-pyrrole group of non-steroidal anti-inflammatory drugs (NSAIDs). Ketorolac Tromethamine Injection, USP is available for intravenous (IV) or intramuscular (IM) administration as: 30 mg in 1 mL (3%) in sterile solution; 60 mg in 2 mL (3%) of ketorolac tromethamine in sterile solution is available for IM administration only.
MAXROLAC 60 is indicated for the short-term (up to 5 days) management of moderately severe, acute pain that requires analgesia at the opioid level, usually in a postoperative setting. It is NOT indicated for minor or chronic painful conditions.
2. What you need to know before you take MAXROLAC 60
Warnings
The most serious risks associated with ketorolac tromethamine are:
GASTROINTESTINAL RISK
Ketorolac tromethamine can cause peptic ulcers, gastrointestinal bleeding and/or perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms.
Therefore, ketorolac tromethamine is contraindicated in patients with active peptic ulcer disease, in patients with recent gastrointestinal bleeding or perforation, and in patients with a history of peptic ulcer disease or gastrointestinal bleeding. Elderly patients are at greater risk for serious gastrointestinal events
CARDIOVASCULAR THROMBOTIC EVENTS
Nonsteroidal anti-inflammatory drugs (NSAIDs) can cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use.
RENAL RISK
Ketorolac tromethamine is contraindicated in patients with advanced renal impairment and in patients at risk for renal failure due to volume depletion.
RISK OF BLEEDING
Ketorolac tromethamine inhibits platelet function and is, therefore, contraindicated in patients with suspected or confirmed cerebrovascular bleeding, patients with hemorrhagic diathesis, incomplete hemostasis and those at high risk of bleeding.
HYPERSENSITIVITY
Hypersensitivity reactions, ranging from bronchospasm to anaphylactic shock, have occurred and appropriate counteractive measures must be available when administering the first dose of ketorolac tromethamine injection. Ketorolac tromethamine is contraindicated in patients with previously demonstrated hypersensitivity to ketorolac tromethamine or allergic manifestations to aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs).
INTRATHECAL OR EPIDURAL ADMINISTRATION
Ketorolac tromethamine is contraindicated for intrathecal or epidural
administration due to its alcohol content.
RISK DURING LABOR AND DELIVERY
The use of ketorolac tromethamine in labor and delivery is contraindicated because it may adversely affect fetal circulation and inhibit uterine contractions.
CONCOMITANT USE WITH NSAIDS
Ketorolac tromethamine is contraindicated in patients currently receiving aspirin or NSAIDs because of the cumulative risk of inducing serious NSAID-related side effects.
SPECIAL POPULATIONS
Dosage should be adjusted for patients 65 years or older, for patients under 50 kg of body weight and for patients with moderately elevated serum creatinine. Doses of ketorolac tromethamine injection are not to exceed 60 mg (total dose per day) in these patients.
Precautions
•Hepatic Effects: Ketorolac tromethamine should be used with caution in patients with impaired hepatic function, or a history of liver disease. Treatment with ketorolac tromethamine may cause elevations of liver enzymes, and in patients with pre-existing liver dysfunction it may lead to the development of a more severe hepatic reaction. The administration of ketorolac tromethamine should be discontinued in patients in whom an abnormal liver test has occurred as a result of ketorolac tromethamine therapy.
•Hematologic Effects: Ketorolac tromethamine inhibits platelet aggregation and may prolong bleeding time; therefore, it is contraindicated as a pre-operative medication, and caution should be used when hemostasis is critical. Unlike aspirin, the inhibition of platelet function by ketorolac tromethamine disappears within 24 to 48 hours after the drug is discontinued. Ketorolac tromethamine does not appear to affect platelet count, prothrombin time (PT) or partial thromboplastin time (PTT). In controlled clinical studies, where ketorolac tromethamine was administered intramuscularly or intravenously postoperatively, the incidence of clinically significant postoperative bleeding was 0.4% for ketorolac tromethamine compared to 0.2% in the control groups receiving narcotic analgesics.
Drug Interactions
Warfarin, Digoxin, Salicylate and Heparin
The in vitro binding of warfarin to plasma proteins is only slightly reduced by ketorolac tromethamine (99.5% control vs 99.3%) when ketorolac plasma concentrations reach 5 to 10 mcg/mL. Ketorolac does not alter digoxin protein binding. In vitro studies indicate that, at therapeutic concentrations of salicylate (300 mcg/mL), the binding of ketorolac was reduced from approximately 99.2% to 97.5%, representing a potential two-fold increase in unbound ketorolac plasma levels. Therapeutic concentrations of digoxin, warfarin, ibuprofen, naproxen, piroxicam, acetaminophen, phenytoin, and tolbutamide did not alter ketorolac tromethamine protein binding. Although the research results do not indicate a significant interaction between ketorolac tromethamine and warfarin or heparin, the administration of ketorolac tromethamine to patients taking anticoagulants should be done extremely cautiously, and patients should be closely monitored.
Furosemide
Ketorolac tromethamine administered IV or IM reduced the diuretic response to furosemide in normovolemic healthy subjects by approximately 20% (mean sodium and urinary output decreased 17%).
Probenecid
Concomitant administration of ketorolac tromethamine tablets and probenecid resulted in decreased clearance of ketorolac and significant increases in ketorolac plasma levels (total AUC increased approximately 3-fold from 5.4 to 17.8 mcg/h/mL) and terminal half-life increased approximately 2-fold from 6.6 to 15.1 hours. Therefore, concomitant use of ketorolac tromethamine and probenecid is contraindicated.
Lithium
Inhibition of renal lithium clearance, leading to an increase in plasma lithium concentration, has been reported with some prostaglandin synthesis-inhibiting drugs. The effect of ketorolac tromethamine on plasma lithium has not been studied, but cases of increased lithium plasma levels during ketorolac tromethamine therapy have been reported.
Methotrexate
Concomitant administration of methotrexate and some NSAIDs has been
reported to reduce the clearance of methotrexate, enhancing the toxicity of methotrexate. The effect of ketorolac tromethamine on methotrexate clearance has not been studied.
Nondepolarizing Muscle Relaxants
In postmarketing experience, there have been reports of a possible interaction between ketorolac tromethamine injection and nondepolarizing muscle relaxants that resulted in apnea. The concurrent use of ketorolac tromethamine with muscle relaxants has not been formally studied.
ACE Inhibitors
Concomitant use of ACE inhibitors may increase the risk of renal impairment, particularly in volume-depleted patients.
Antiepileptic Drugs
Sporadic cases of seizures have been reported during concomitant use of ketorolac tromethamine and antiepileptic drugs (phenytoin, carbamazepine). Psychoactive Drugs
Hallucinations have been reported when ketorolac tromethamine was used in patients taking psychoactive drugs (fluoxetine, thiothixene, alprazolam). Morphine
Ketorolac tromethamine injection has been administered concurrently with morphine in several clinical trials of postoperative pain without evidence of adverse interactions. Do not mix ketorolac tromethamine and morphine in the same syringe. There is no evidence in animal or human studies that ketorolac tromethamine induces or inhibits hepatic enzymes capable of metabolizing itself or other drugs.
Pregnancy and Lactation
Ketorolac tromethamine should not be used during pregnancy and lactation.
DIRECTION
1.How to take MAXROLAC 60
Ketorolac tromethamine injection may be used as a single or multiple dose. When administering this medication, the IV bolus must be given over no less than 15 seconds. The IM administration should be given slowly and deeply into the muscle.
Single-Dose Treatment: The following regimen should be limited to single administration use only.
Adult Patients:
IM Dosing:
•Patients less than 65 years of age: One dose of 60 mg.
•Patients greater than or equal to 65 years of age, renally impaired and/or less than 50 kg of body weight: One dose of 30 mg.
IV Dosing:
•Patients less than 65 years of age: One dose of 30 mg.
•Patients greater than or equal to 65 years of age, renally impaired and/or less than 50 kg of body weight: One dose of 15 mg.
Pediatric Patients (2 to 16 years of age): The pediatric population should receive only a single dose of ketorolac tromethamine injection as follows:
IM Dosing:
One dose of 1 mg/kg up to a maximum of 30 mg. IV Dosing:
One dose of 0.5 mg/kg up to a maximum of 15 mg.
Multiple-Dose Treatment (IV or IM):
•Patients less than 65 years of age: The recommended dose is 30 mg ketorolac tromethamine injection every 6 hours. The maximum daily dose should not exceed 120 mg.
•For patients greater than or equal to 65 years of age, renally impaired patients, and patients less than 50 kg: The recommended dose is 15 mg ketorolac tromethamine injection every 6 hours. The maximum daily dose for these populations should not exceed 60 mg.
2.Possible side effects
Adverse reaction rates increase with higher doses of ketorolac tromethamine. The Adverse Reactions Listed Below Were Reported In Clinical Trials As Probably Related To KETOROLAC TROMETHAMINE.
• Incidence Greater Than 1% Body as a Whole: edema. Cardiovascular: hypertension. Dermatologic: pruritus, rash.
Gastrointestinal: nausea, dyspepsia, gastrointestinal pain, diarrhea,
constipation, flatulence, gastrointestinal fullness, vomiting, stomatitis. Hemic and Lymphatic: purpura.
Nervous System: headache, drowsiness, dizziness, sweating. Injection-site pain was reported by 2% of patients in multi-dose studies.
• Incidence 1% or Less
Body as a Whole: weight gain, fever, infections, asthenia.
Cardiovascular: palpitation, pallor, syncope.
Dermatologic: urticaria.
Gastrointestinal: gastritis, rectal bleeding, eructation, anorexia, increased appetite.
Hemic and Lymphatic: epistaxis, anemia, eosinophilia.
Nervous System: tremors, abnormal dreams, hallucinations, euphoria, extrapyramidal symptoms, vertigo, paresthesia, depression, insomnia, nervousness, excessive thirst, dry mouth, abnormal thinking, inability to concentrate, hyperkinesis, stupor.
Respiratory: dyspnea, pulmonary edema, rhinitis, cough.
Special Senses: abnormal taste, abnormal vision, blurred vision, tinnitus, hearing loss.
Urogenital: hematuria, proteinuria, oliguria, urinary retention, polyuria, increased urinary frequency.
The Following Adverse Events Were Reported From Postmarketing Experience. Body as a Whole: hypersensitivity reactions such as anaphylaxis, anaphylactoid reaction, laryngeal edema, tongue edema, angioedema, myalgia.
Cardiovascular: hypotension and flushing.
Dermatologic: Lyell’s syndrome, Stevens-Johnson syndrome, exfoliative dermatitis, maculo-papular rash, urticaria.
Gastrointestinal: peptic ulceration, G.I. hemorrhage, G.I. perforation, melena, acute pancreatitis, hematemesis, esophagitis.
Hemic and Lymphatic: postoperative wound hemorrhage rarely requiring blood transfusion, thrombocytopenia, leukopenia.
Hepatic: hepatitis, liver failure, cholestatic jaundice.
Nervous System: convulsions, psychosis, aseptic meningitis.
Respiratory: asthma, bronchospasm.
Urogenital: acute renal failure (see Boxed WARNING, WARNINGS), flank pain with or without hematuria and/or azotemia, nephritis, hyponatremia, hyperkalemia, hemolytic uremic syndrome.
INGREDIENTS
Contents of the pack and other information
What MAXROLAC 60 contains
•Each ampoule of the active substance is Ketorolac Tromethamine.
•The other ingredients are: alcohol, propylene glycol, sodium chloride, sodium hydroxide and/or hydrochloride acid and water for injections.
What MAXROLAC 60 looks like and contents of the pack
•MAXROLAC 60 is a clear and colorless or slightly yellow solution in color, free from visible particles.
•Pack size:
Ketorolac Tromethamine Injection, USP for intramuscular and intravenous use: 30 mg: 30 mg/mL, available in 1 mL ampoules, 5 ampoules per tray, 2 trays per carton.
Ketorolac Tromethamine Injection, USP for intramuscular use only:
60 mg: 30 mg/mL, available in 2 mL ampoules, 5 ampoules per tray, 2 trays per carton.
STORE
How to store MAXROLAC 60
Keep this medicine out of the sight and reach of children.
Store at temperature not exceeding 25°C. Do not refrigerate or freeze. Keep the ampoules in the outer carton until time of use in order to protect from light.
Do not use this medicine after the expiry date ‘EXP’ shown on the container. The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater or household. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.