Ceftriaxone for Injection,USP
QUICK DETAILS:
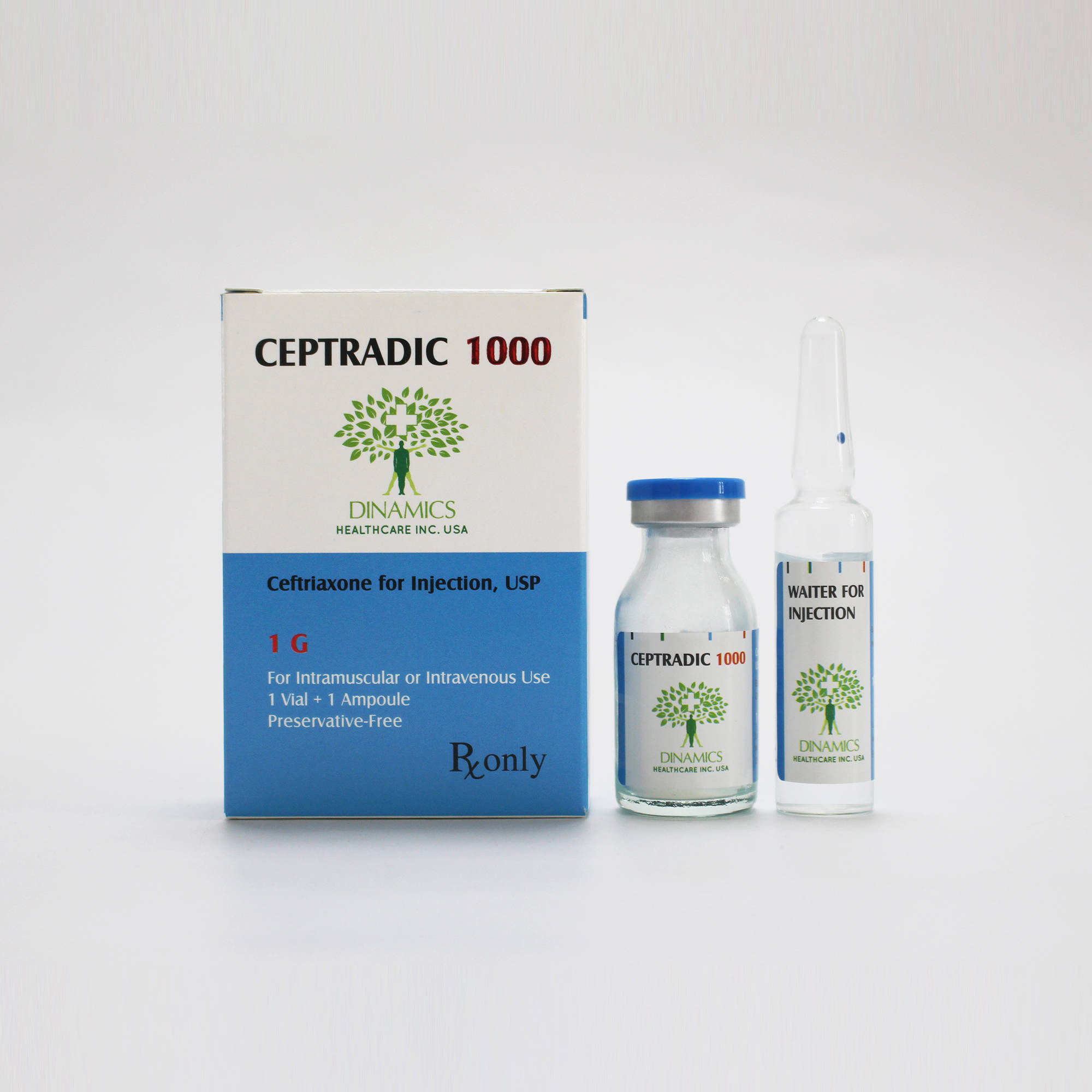
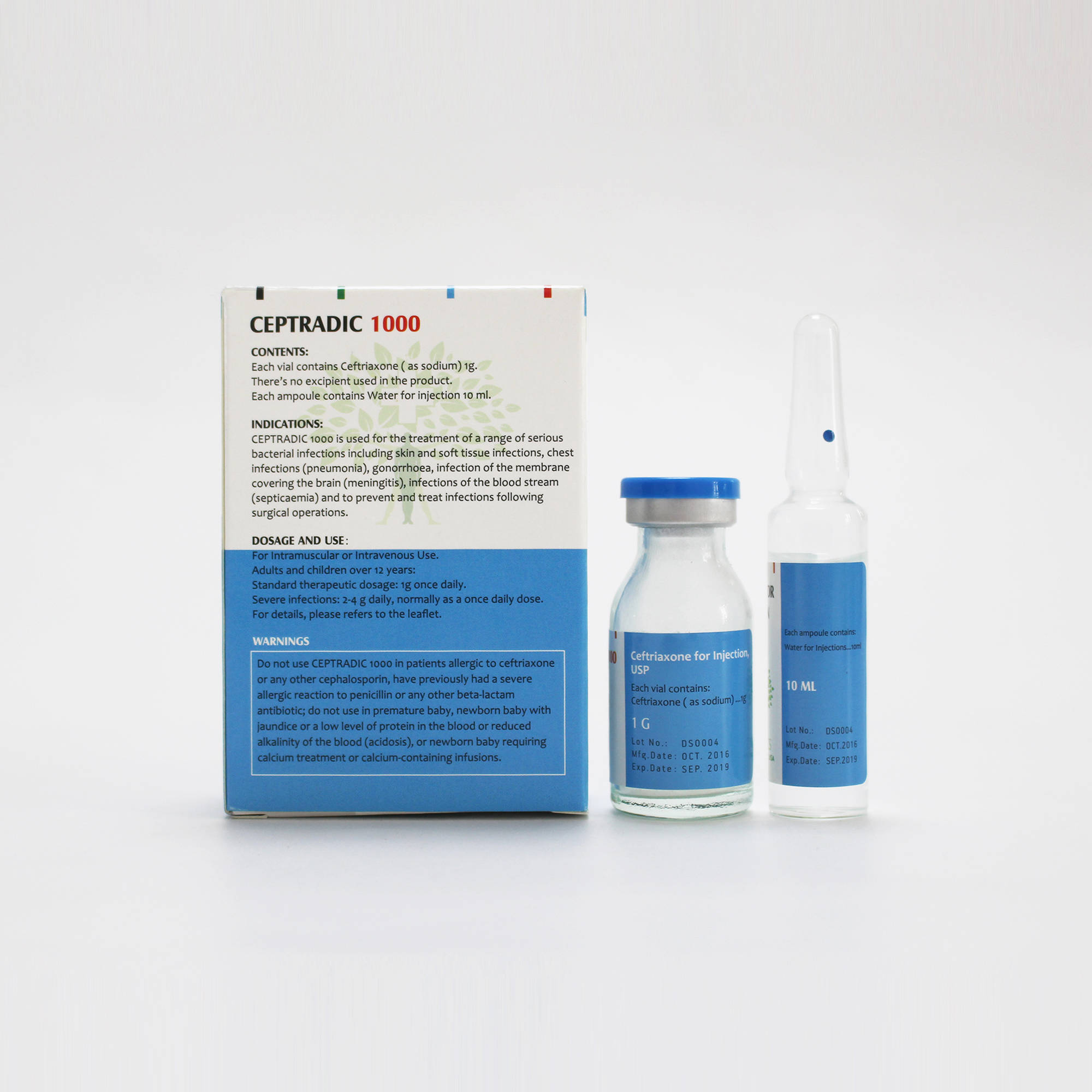
CEPTRADIC 1000 Contents:
• Each vial contains ceftriaxone ( as sodium) 1g as the active ingredient.
• There’s no excipient used in the product.
• Each ampoule contains 10ml of water for injection as the solvent.
CEPTRADIC 1000 Character:
CEPTRADIC 1000 is a white or almost white crystalline powder.
CEPTRADIC 1000 Pack:
1 vial and 1 ampoule per box.
DESCRIPTION
1. What CEPTRADIC 1000 is and what it is used for
Ceftriaxone, the active ingredient of CEPTRADIC 1000, belongs to a group of medicines called cephalosporins, which are antibiotics. These medicines work by killing bacteria that cause infections.
CEPTRADIC 1000 is used for the treatment of a range of serious bacterial infections including skin and soft tissue infections, chest infections (pneumonia), gonorrhoea, infection of the membrane covering the brain (meningitis), infections of the blood stream (septicaemia) and to prevent and treat infections following surgical operations
2. What you need to know before you take CEPTRADIC 1000
CEPTRADIC 1000 should not be given if:
•you are allergic to ceftriaxone or any other cephalosporin.
•you have previously had a severe allergic reaction to penicillin or any other beta-lactam antibiotic.
•the patient is a premature baby
•the patient is a newborn baby with jaundice or a low level of protein in the blood or reduced alkalinity of the blood (acidosis)
•the patient is a newborn baby requiring calcium treatment or
calcium-containing infusions.
Take special care with CEPTRADIC 1000 if:
•you have previously had an allergic reaction to penicillin or other antibiotics of this type. Not all people who are allergic to penicillins are also allergic to cephalosporins. Before you are given this medicine your doctor should check whether you have previously had an allergic reaction to such drugs.
•the patient is a baby or small child who needs high doses of ceftriaxone.
•you have kidney and liver problems, if you have recently been very ill or if you are being fed through a vein.
•you are on a low sodium diet.
•you are going to have a blood transfusion, make sure that the doctor who organises your transfusion knows that you are having CEPTRADIC 1000.
•you are diabetic, you may get false positive results with urine glucose tests, such as Clinitest.
•ceftriaxone can cause bleeding, particularly in patients who are malnourished, have kidney or liver problems or have low vitamin K levels.
•you have anaemia
•you have diarrhoea or other bowel related problems
•you have or have a history of colitis (inflamed colon)
You should be kept under observation in case you develop another infection, particularly colitis (infection of the lower bowel), while you are being treated with CEPTRADIC 1000.
You should let your doctor or nurse know if you have recently received or are about to receive calcium. Patients given high doses of CEPTRADIC 1000 may develop calcium precipitates in the gall bladder.
Taking other medicines
Taking other medicines when CEPTRADIC 1000 is being administered can affect how it or the other medicine works. Make sure that your doctor knows what other medicines you are taking. Do not take any other medicines while you are being treated with CEPTRADIC 1000 unless you have told your doctor or pharmacist and asked their advice. This includes medicines you may have bought yourself without a prescription.
Please check with your doctor if you are taking the following (or any other medication):
•Antibiotics such as vancomycin and chloramphenicol.
•Anticoagulants (medicines used to stop blood clotting) such as warfarin.
•Ceftriaxone should not be administered simultaneously with intravenous calcium-containing solutions.
•Oral contraceptives as ceftriaxone may effect their function. You should use non-hormonal contraceptives during treatment and in the month following treatment.
•Amsacrine, used in the treatment of leukemia.
•Fluconazole, an antifungal used in the treatment of thrush.
If you have any doubts about whether you should be given this medicine, then talk to your doctor.
Important information about the sodium content of CEPTRADIC 1000
CEPTRADIC 1000 contains 3.6mmol (or 82mg) for the 1g vial sodium per dose. To be taken into consideration by patients on a controlled sodium diet.
Pregnancy and breast-feeding
Ceftriaxone crosses the placental barrier. Safety in human pregnancy has not been established. Low concentrations of ceftriaxone are excreted in human milk. for the sake of safety, this medicine should not be used in pregnancy and breast-feeding women unless absolutely indicated.
You should let your doctor or nurse know if you are pregnant or wish to become pregnant or are breast-feeding before this medicine is administered.
Driving and using machines
CEPTRADIC 1000 may cause dizziness. If you are affected you should not drive or operate machinery.
DIRECTION
1.How to take CEPTRADIC 1000
Intramuscular or Intravenous route.
Intramuscular injection: 1g ceftriaxone should be dissolved in 5ml of Water for Injection. Doses greater than 1g should be divided and injected at more than one site.
Intravenous injection: 1g ceftriaxone should be dissolved in 10ml of Water for Injection. The injection should be administered over at least 2 - 4 minutes, directly into the vein.
Intravenous infusion: 1g of ceftriaxone should be dissolved in 20ml of one of the following calcium-free solutions:
Dextrose Injection 5% or 10%, Sodium Chloride Injection, Sodium Chloride and Dextrose Injection (0.45% Sodium Chloride and 2.5% Dextrose), Dextran 6% in Dextrose Injection 5%, Hydroxyethyl Starch 6 - 10% infusions. The infusion should be administered over at least 30 minutes.
Adults and children over 12 years:
Standard therapeutic dosage: 1g once daily.
Severe infections: 2-4 g daily, normally as a once daily dose.
The duration of therapy varies according to the course of the disease. As with antibiotic therapy in general, administration of ceftriaxone should be continued for a minimum of 48 to 72 hours after the patient has become afebrile or evidence of bacterial eradication has been obtained.
Acute, uncomplicated gonorrhoea: one dose of 250mg intramuscularly should be administered. Simultaneous administration of probenecid is not indicated.
Peri-operative prophylaxis: usually one dose of 1g given . In colorectal surgery, 2g should be given, in conjunction with a suitable agent against anaerobic bacteria.
Elderly:
These dosages do not require modification in elderly patients provided that renal and hepatic function are satisfactory .
In the neonate, the intravenous dose should be given over 60 minutes to reduce the displacement of bilirubin from albumin, thereby reducing the potential risk of bilirubin encephalopathy.
Children under 12 years
Standard therapeutic dosage: 20-50mg/kg body-weight once daily.
Up to 80mg/kg body-weight daily may be given in severe infections, except in premature neonates where a daily dosage of 50mg/kg should not be exceeded. For children with body weights of 50kg or more, the usual dosage should be used. Doses of 50mg/kg or over should be given by slow intravenous infusion over at least 30 minutes. Doses greater than 80mg/kg body weight should be avoided because of the increased risk of biliary precipitates.
Renal and hepatic impairment: In patients with impaired renal function, there is no need to reduce the dosage of ceftriaxone provided liver function is intact. Only in cases of pre-terminal renal failure (creatinine clearance <10ml per minute) should the daily dosage be limited to 2g or less.
In patients with liver damage there is no need for the dosage to be reduced provided renal function is intact.
In severe renal impairment accompanied by hepatic insufficiency, the plasma concentration of ceftriaxone should be determined at regular intervals and dosage adjusted.
In patients undergoing dialysis, no additional supplementary dosage is required following the dialysis. Plasma concentrations should be monitored, however, to determine whether dosage adjustments are necessary, since the elimination rate in these patients may be reduced.
2. Possible side effects
Like all medicines, CEPTRADIC 1000 can cause side effects, although not everybody gets them. The following side effects may happen with this medicine.
These include:
• Allergic reactions, with skin rash, itching and swelling of the eyes and face. More severe allergic reactions may be associated with headache, fever, shivering, wheezing, aching joints, sore eyes, or red, blistering and peeling skin.
Tell your doctor immediately if you think you are having an allergic reaction to ceftriaxone.
•The most common side effects are loose stools and diarrhoea or, occasionally nausea and vomiting or a sore mouth and tongue.
•Some other side effects patients have had with ceftriaxone include headache and anaemia or other changes in the blood (causing sore throat and mouth ulcers or a tendency to bleed or bruise easily).
•Rarely, dizziness, vertigo, stomach pain, disturbances of liver or kidney function, hepatitis, jaundice, blood or glucose in the urine or a fungal infection of the genital tract (thrush) can occur. Deposits in the urinary passages or gall bladder may cause problems with passing water, passing little or no water, or stomach pains, especially in very young, dehydrated or bedridden patients. These deposits usually disappear when ceftriaxone treatment is stopped.
•Antibiotic treatment can affect the normal bacteria in the gut, causing new infection (colitis). You should tell your doctor immediately if you develop diarrhoea after starting treatment with ceftriaxone.
•Occasionally, if you have had an intravenous injection there may be soreness or swelling around the area of injection.
INGREDIENTS
Contents of the pack and other information
What CEPTRADIC 1000 contains
• Each vial contains ceftriaxone ( as sodium) 1g as the active ingredient.
• There’s no excipient used in the product.
• Each ampoule contains 10ml of water for injection as the solvent.
What CEPTRADIC 1000 looks like and contents of the pack
CEPTRADIC 1000 is a white or almost white crystalline powder. 1 vial and 1 ampoule per box.
STORE
How to store CEPTRADIC 1000
CEPTRADIC 1000 must not be used after the expiry date ‘EXP’ shown on the container.
Keep out of the reach and sight of children. Do not store above 25°C.
After reconstitution: Store at 2-8°C. Do not freeze.