Citicoline Injection,USP
QUICK DETAILS:
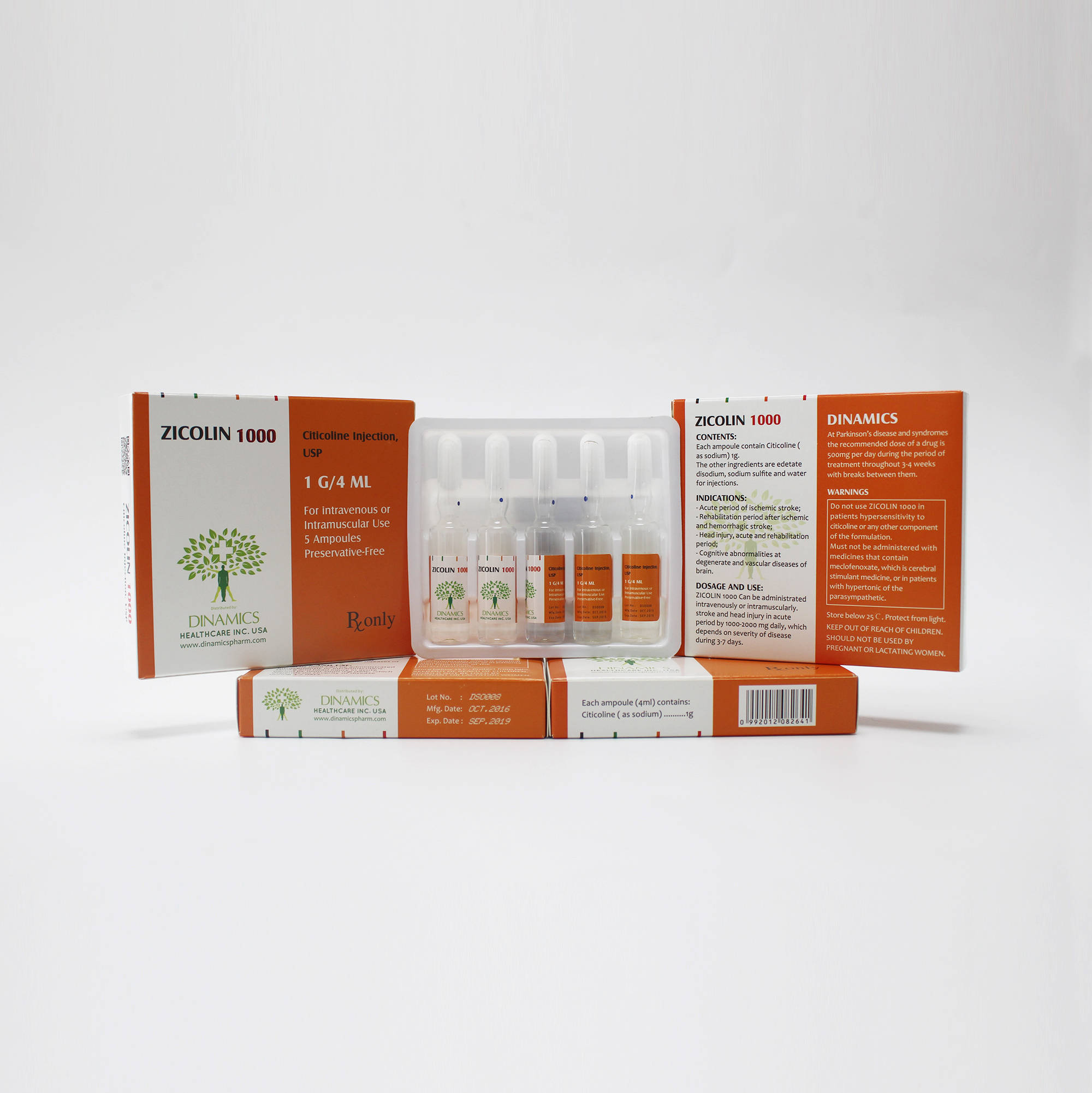
ZICOLIN 1000 Content:
• Each ampoule contain 1g of Citicoline ( as sodium ) as the active ingredient.
• The other ingredients are edetate disodium, sodium sulfite and water for injections.
ZICOLIN 1000 Character:
ZICOLIN 1000 is a clear, colorless solution which comes in a glass ampoule containing 4 ml of solution.
ZICOLIN 1000 Pack:
5 ampoules in a plastic tray, 1 tray per box.
DESCRIPTION
1.What ZICOLIN 1000 is and what it is used for
ZICOLIN 1000 contains the active substance Citicoline which is a complex organic molecule that functions as an intermediate in the biosynthesis of cell membrane phospholipids.
ZICOLIN 1000 is indicated for:
- Acute period of ischemic stroke;
- Rehabilitation period after ischemic and hemorrhagic stroke;
- Head injury, acute and rehabilitation period;
- Cognitive abnormalities at degenerate and vascular diseases of brain.
2.What you need to know before you take ZICOLIN 1000
CONTRAINDICATIONS:
Hypersensitivity to citicoline or any other component of the formulation. Must not be administered to patients with hypertonic of the parasympathetic. Do not use in pregnancy and lactation.
Warnings and precautions General
For the acute, severe case of progressive disturbance of consciousness, antihemorrhagics or brain pressure-decreasing agents should be given concomitantly and the body temperature maintained at a low level.
For patients with disturbance of consciousness in acute stage of cerebral infarction, start of citicoline injection is recommended within 2 weeks after apoplectic stroke.
In administering citicoline injection intramuscularly, caution should be exercised so as not to affect the tissues, nerves, etc. Intramuscular injection should be given only when indispensable,and should be restricted to the minimum to be required. In particular, repeated injection at the same site should be avoided.Care should be exercised to avoid injection at sites along the course of the nerves. In case of intense pain or backflow of blood upon insertion of the injection needle, the needle should be withdrawn immediately and injected at a different site.In intravenous administration, inject as slowly as possible.Since shock may occur, a close observation should be maintained. If any such abnormalities such as drop in bloodpressure, distressed feeling of the chest or dyspnoea areobserved, citicoline injection should be discontinued and appropriate measures taken.
Drug Interaction
Citicoline enhances the effects of L-Dopa and must not be administered together with medicines that contain L-Dopa without medical consultation. Furthermore, ZICOLIN 1000 must not be administered with medicines that contain meclofenoxate, which is cerebral stimulant medicine.
Pregnancy and Lactation
There are no adequate and well controlled studies of citicoline during pregnancy and lactation. Citicoline should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Caution should be exercised during breast feeding because it is not known whether citicoline is excreted in human breast milk.
Driving and using machinery
Patients are advised not to driving and operate heavy machinery or automobiles until the full effect of Citicoline is known.
DIRECTION
1.How to take ZICOLIN 1000
ZICOLIN 1000 Can be administrated intravenously or intramuscularly.
It is prescribed intravenously in the form of a bolus intravenous injection (during 5 minutes) or slow intravenous infusion (40-60 drops per minute) at stroke and head injury in acute period by 1000-2000 mg daily, which depends on severity of disease during 3-7 days with the subsequent change to intramuscular administration or oral administration. The intravenous way of administration is more preferable, than intramuscular.
intramuscularly: 1-2 injections per day. It is necessary to avoid repeated administration of the drug to the same place during intramuscular administration.
At long-term impairment of consciousness continuous application of drug from the first stages of disease is possible.
At Parkinson’s disease and syndromes the recommended dose of a drug is 500mg per day during the period of treatment throughout 3-4 weeks with breaks between them.
2. Possible side effects
Like all medicines, ZICOLIN 1000 can cause side effects, although not everybody gets them. The following side effects may happen with this medicine.
From CNS and peripheral nervous system: sleeplessness, headache, dizziness, excitation, tremor, numbness in the paralyzed limbs.
From digestive system: nausea, appetite decrease, change of activity of hepatic enzymes.
Allergic reactions: rash, skin itch, anaphylactic shock.
The others: heat; in some cases - short-term hypotensive action, stimulation of parasympathetic nervous system
INGREDIENTS
Contents of the pack and other information
What ZICOLIN 1000 contains
•Each ampoule contain 1g of Citicoline ( as sodium ) as the active ingredient.
•The other ingredients are edetate disodium, sodium sulfite and water for injections.
What ZICOLIN 1000 looks like and contents of the pack
ZICOLIN 1000 is a clear, colorless solution which comes in a glass ampoule containing 4 ml of solution.
5 ampoules in a plastic tray, 1 tray per box.
STORE
How to store ZICOLIN 1000
ZICOLIN 1000 must not be used after the expiry date ‘EXP’ shown on the container.
Keep out of the reach and sight of children. Store below 250C, protect from light.