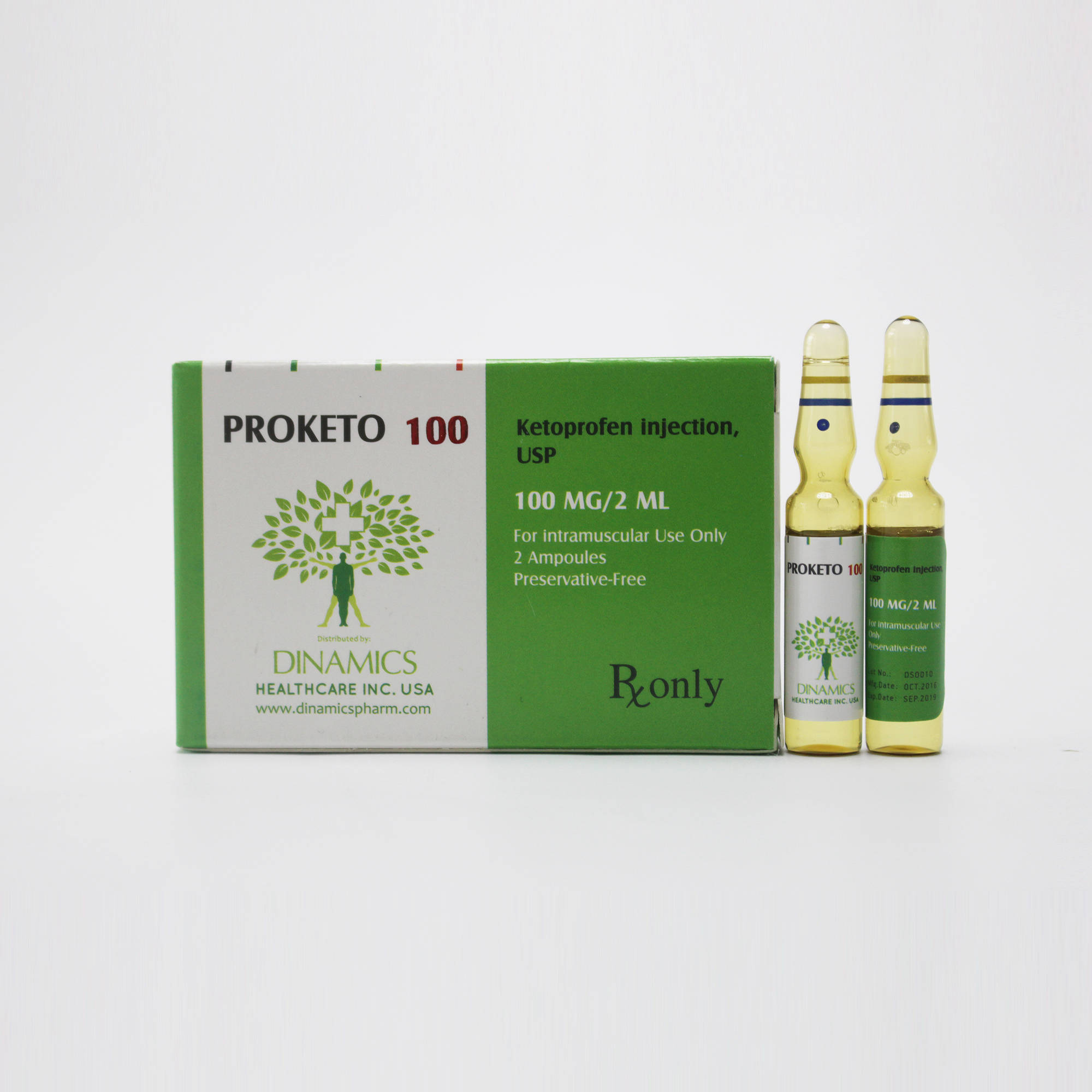
1. What PROKETO 100 is and what it is used for
PROKETO 100 contains the active substance Ketoprofen which is one of a group of medicines called non-steroidal anti-inflammatory drugs (NSAIDS).
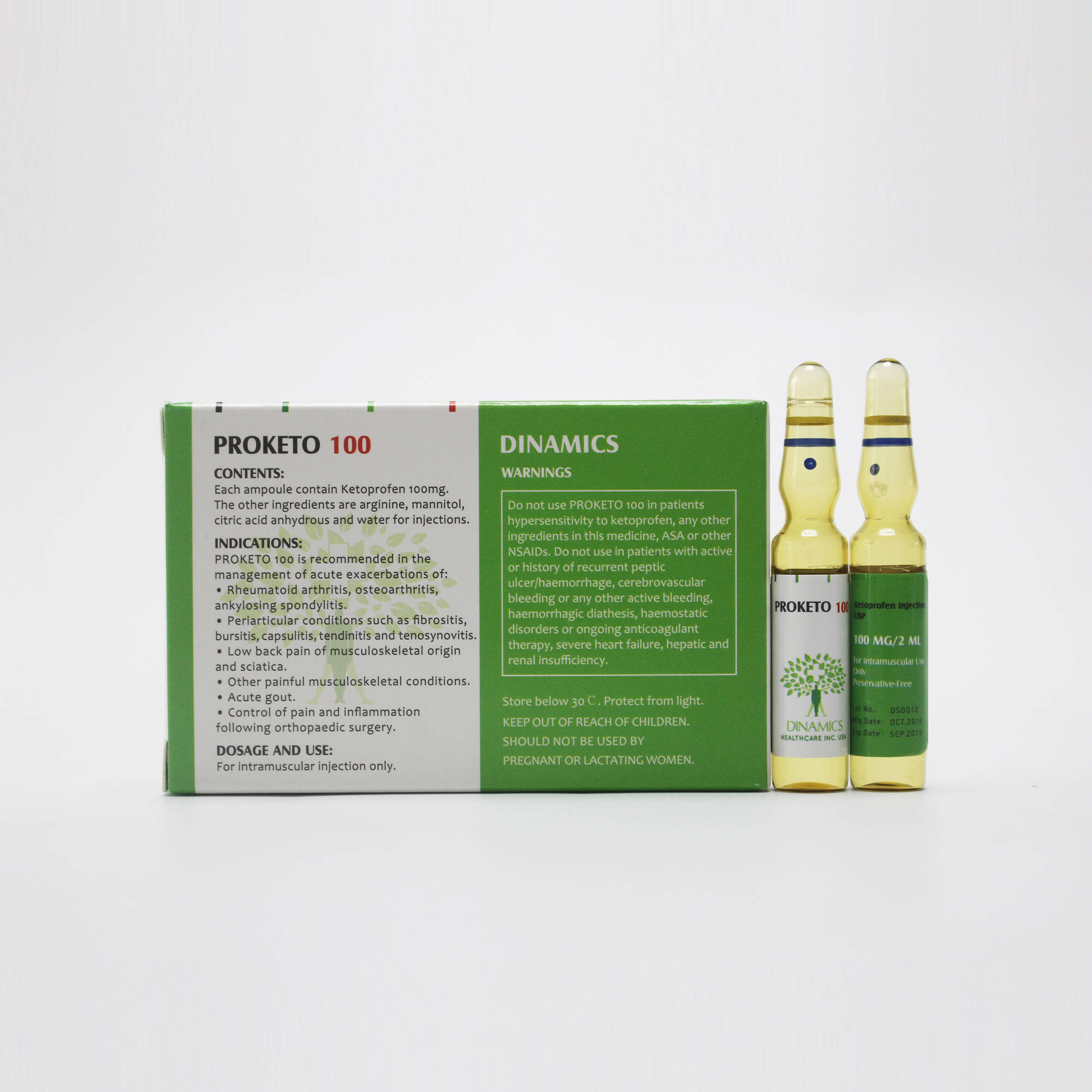
PROKETO 100 is recommended in the management of acute exacerbations of:
•Rheumatoid arthritis, osteoarthritis, ankylosing spondylitis.
•Periarticular conditions such as fibrositis, bursitis, capsulitis, tendinitis and tenosynovitis.
•Low back pain of musculoskeletal origin and sciatica.
•Other painful musculoskeletal conditions.
•Acute gout.
•Control of pain and inflammation following orthopaedic surgery.
2. What you need to know before you take PROKETO 100
Contraindications
PROKETO 100 is contraindicated in:
-Patients who have a history of hypersensitivity reactions such as bronchospasm, asthmatic attacks, rhinitis, angioedema, urticaria or other allergic-type reactions to ketoprofen, any other ingredients in this medicine, ASA or other NSAIDs.
-Pregnancy and lactation
-Patients with severe heart failure
-Patients with active or history of recurrent peptic ulcer/haemorrhage (two or more distinct episodes of proven ulceration or bleeding)
-Patients with haemorrhagic diathesis
-Patients with severe hepatic insufficiency
-Patients with severe renal insufficiency
-Cases of cerebrovascular bleeding or any other active bleeding.
-Patients with haemostatic disorders or ongoing anticoagulant therapy.
Special Warnings And Precautions For Use
PROKETO 100 is for intramuscular use only.
Undesirable effects may be minimised by using the lowest effective dose for the shortest duration necessary to control symptoms.
The use of ketoprofen with concomitant NSAIDs including cyclooxygenase-2 selective inhibitors should be avoided.
Elderly:
The elderly have an increased risk of adverse reactions to NSAIDs, especially gastrointestinal bleeding and perforation which may be fatal.
Cardiovascular, Renal and Hepatic impairment:
At the start of treatment, renal function must be carefully monitored in patients with heart impairment, heart failure, liver dysfunction, cirrhosis and nephrosis, in patients receiving diuretic therapy, in patients with chronic renal impairment, particularly if the patient is elderly. In these patients, administration of ketoprofen may induce a reduction in renal blood flow caused by prostaglandin inhibition and lead to renal decomposition.
Cardiovascular and cerebrovascular effects
Appropriate monitoring and advice are required for patients with a history of hypertension and/or mild to moderate congestive heart failure as fluid retention and oedema have been reported in association with NSAID therapy.
Clinical trial and epidemiological data suggest that use of some NSAIDs (particularly at high doses and in long-term treatment) may be associated with a small increased risk of arterial thrombotic events (for example myocardial infarction or stroke). There are insufficient data to exclude such a risk for ketoprofen.
Respiratory disorders:
Patients with asthma combined with chronic rhinitis, chronic sinusitis, and/or
nasal polyposis have a higher risk of allergy to aspirin and/or NSAIDs than the rest of the population. Administration of this medicinal product can cause asthma attacks or bronchospasm, particularly in subjects allergic to aspirin or NSAIDs
Gastrointestinal bleeding, ulceration and perforation:
GI bleeding, ulceration or perforation, which can be fatal, has been reported with all NSAIDs at any time during treatment, with or without warning symptoms or a previous history of serious GI events.
Some epidemiological evidence suggests that ketoprofen may be associated with a high risk of serious gastrointestinal toxicity, relative to some other NSAIDs, especially at high doses
The risk of GI bleeding, ulceration or perforation is higher with increasing NSAlD doses, in patients with a history of ulcer, particularly if complicated with haemorrhage or perforation, and in the elderly. These patients should commence treatment on the lowest dose available. Combination therapy with protective agents (e.g. misoprostol or proton pump inhibitors) should be considered for these patients, and also for patients requiring concomitant low dose aspirin, or other drugs likely to increase gastrointestinal risk. NSAIDs should only be given with care to patients with a history of gastrointestinal disease (e.g. ulcerative colitis, Crohn's disease) as these conditions may be exacerbated.
Patients with a history of gastrointestinal toxicity, particularly when elderly, should report any unusual abdominal symptoms (especially GI bleeding), particularly in the initial stages of treatment.
Caution should be advised in patients receiving concomitant medications which could increase the risk of ulceration or bleeding, such as oral corticosteroids, anticoagulants such as warfarin, selective serotonin-reuptake inhibitors or anti-platelet agents such as aspirin.
When GI bleeding or ulceration occurs in patients receiving ketoprofen, the treatment should be withdrawn.
SLE and mixed connective tissue disease:
In patients with systemic lupus erythematosis (SLE) and mixed connective tissue disorders there may be an increased risk of aseptic meningitis.
Impaired female fertility:
The use of ketoprofen, as with other NSAIDs, may impair female fertility and is not recommended in women attempting to conceive. In women who have difficulties conceiving or who are undergoing investigation of infertility, withdrawal of ketoprofen should be considered.
Skin reactions:
Serious skin reactions, some of them fatal, including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, have been reported very rarely in association with the use of NSAlDs. Patients appear to be at highest risk for these reactions early in the course of therapy, the onset of the reaction occurring in the majority of cases within the first month of treatment. Treatment should be discontinued at the first appearance of skin rash, mucosal lesions, or any other sign of hypersensitivity.
Infectious disease:
As with other NSAIDs, in the presence of an infectious disease, it should be noted that the anti-inflammatory, analgesic and the antipyretic properties of ketoprofen may mask the usual signs of infection progression such as fever. Risk of gastrointestinal bleeding: the relative risk increases in subjects who have a low body weight. If gastrointestinal bleeding or ulcer occur, treatment must be discontinued immediately.
Blood counts and liver and kidney function tests should be carried out during long-term treatment.
Hyperkalaemia:
Hyperkalaemia promoted by diabetes or concomitant treatment with potassium-sparing agents.
Potassium levels must be monitored regularly under these circumstances. In patients with abnormal liver function tests or with a history of liver disease, transaminase levels should be evaluated periodically, particularly during long-term therapy.
Patients with active or a past history of peptic ulcer.
Interaction with other medicinal product and other forms of interaction
As Ketoprofen may cause GI bleeding, inhibit platelet aggregation and prolong bleeding time, the drug should be used with caution and the patient should be carefully observed if the drug is used concomitantly with any anticoagulant or thrombolytic agent. Concomitant administration of Ketoprofen and hydrochlorothiazide has resulted in decreased urinary excretion of potassium and chloride compared with hydrochlorothiazide alone. Ketoprofen and salicylates appear to interact in a complex manner and they should not be used concomitantly. Concomitant use of Ketoprofen and probenecid is also not recommended. Ketoprofen should be avoided in patients receiving methotrexate.
Pregnancy And Lactation
Pregnancy
Inhibition of prostaglandin synthesis may adversely affect the pregnancy
and/or the embryo/foetal development. Data from epidemiological studies suggest an increased risk of miscarriage and of cardiac malformation and gastroschisis after use of a prostaglandin synthesis inhibitor in early pregnancy. The absolute risk for cardiovascular malformation was increased from less than 1%, up to approximately 1.5%. The risk is believed to increase with dose and duration of therapy. In animals, administration of a prostaglandin synthesis inhibitor has been shown to result in increased pre-and post-implantation loss and embryo-foetal lethality. In addition, increased incidences of various malformations, including cardiovascular, have been reported in animals given a prostaglandin synthesis inhibitor during the organogenetic period. During the first and second trimester of pregnancy, ketoprofen should not be given unless clearly necessary. If ketoprofen is used by a woman attempting to conceive, or during the first and second trimester of pregnancy, the dose should be kept as low and duration of treatment as short as possible.
During the third trimester of pregnancy, all prostaglandin synthesis inhibitors may expose the foetus to:
-cardiopulmonary toxicity (with premature closure of the ductus arteriosus and pulmonary hypertension);
-renal dysfunction, which may progress to renal failure with
oligo-hydroamniosis; the mother and the neonate, at the end of the pregnancy, to:
-possible prolongation of bleeding time, an anti-aggregating effect which may occur even at very low doses.
-Inhibition of uterine contractions resulting in delayed or prolonged labour. Consequently, ketoprofen is contraindicated during the third trimester of pregnancy.
Lactation
No data are available on excretion of ketoprofen in human milk. Ketoprofen is not recommended in nursing mothers.
Effects On Ability To Drive And Use Machines
Patients should be warned about the potential for somnolence, dizziness or convulsions and be advised not to drive or operate machinery if these symptoms occur.
Patients should be warned of possible visual disturbances. If patients experience this, they should not drive or use machines.